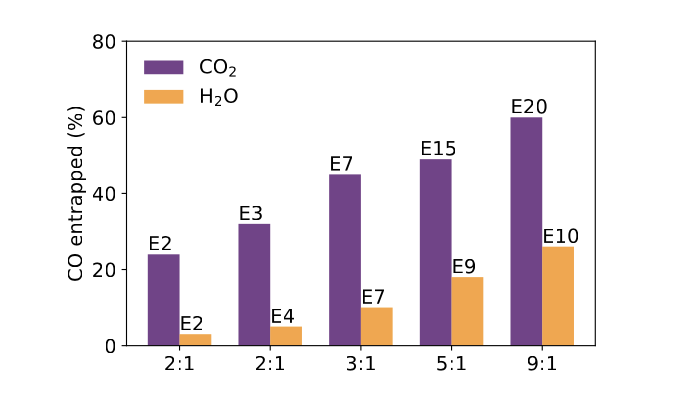
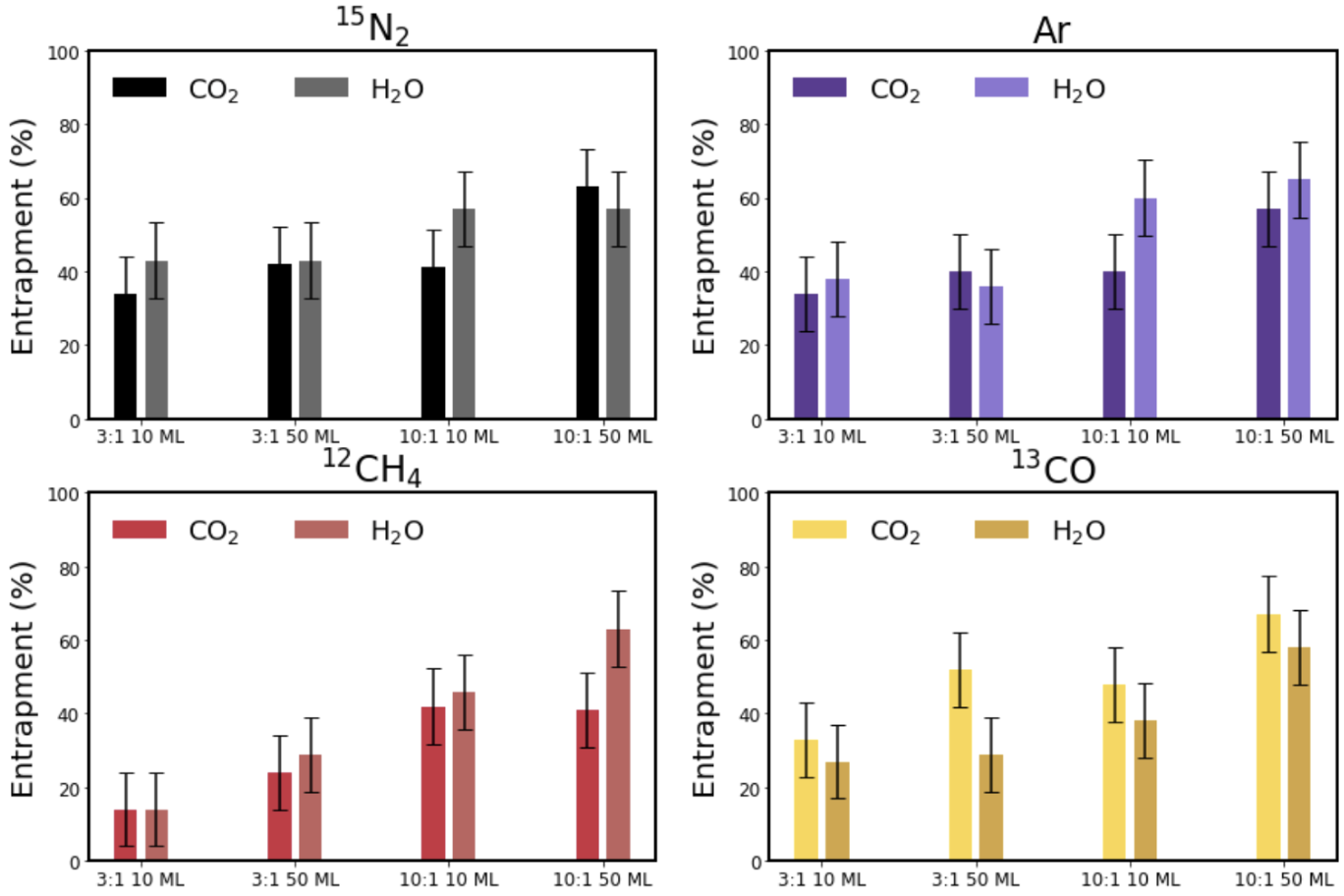
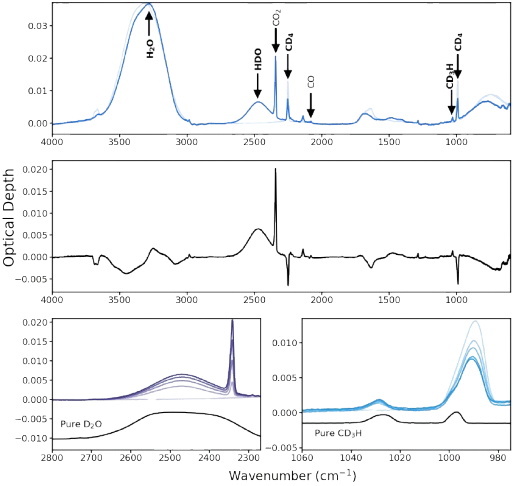
Entrapment of CO in CO2 Ice
Planet atmosphere and hydrosphere compositions are fundamentally set by accretion of volatiles, and therefore by the division of volatiles between gas and solids in planet-forming disks. For hyper-volatiles such as CO, this division is regulated by volatile sublimation energies, and by the ability of other ice components to entrap. Water ice is known for its ability to trap CO and other volatile species. In this study we explore whether another common interstellar and cometary ice component, CO2, is able to trap CO as well. We measure entrapment of CO molecules in CO2 ice through temperature-programmed desorption experiments on CO2:CO ice mixtures. We find that CO22ice traps CO with a typical efficiency of 40%–60% of the initially deposited CO molecules for a range of ice thicknesses between 7 and 50 monolayers, and ice mixture ratios between 1:1 and 9:1. The entrapment efficiency increases with ice thickness and CO dilution. We also run analogous H2O:CO experiments and find that under comparable experimental conditions, CO2 ice entraps CO more efficiently than H2O ice up to the onset of CO2 desorption at ∼70 K. We speculate that this may be due to different ice restructuring dynamics in H2O and CO2 ices around the CO desorption temperature. Importantly, in planet-forming disks, the ability of CO2 to entrap CO may change the expected division between gas and solids for CO and other hyper-volatiles exterior to the CO2 snowline. Read more.